The WISeR Model: CMS's New Weapon Against Waste in Wound Care and Beyond
CMS is launching the WISeR Model in 2026! Learn what this mandatory, AI-driven prior authorization program means for skin substitutes and wound care billing.
admin
11/10/20257 min read
The landscape of Medicare payment for healthcare services is constantly evolving, driven by the persistent challenge of ensuring value-based care while safeguarding the integrity of the Medicare Trust Fund. In this ongoing effort, the Centers for Medicare & Medicaid Services (CMS) has unveiled a new, formidable initiative: the Wasteful and Inappropriate Service Reduction (WISeR) Model.
Launching on January 1, 2026, this mandatory, six-year demonstration model is poised to significantly impact how certain services—including high-cost skin substitutes—are approved and reimbursed. For providers, especially those in advanced wound care, the WISeR Model is not just another regulatory hurdle; it represents a fundamental shift in oversight, demanding meticulous medical necessity documentation and proactive engagement with prior authorization.
Understanding the "what," "why," and "what to expect" of the WISeR Model is no longer optional for affected providers. It is an imperative for maintaining compliance and ensuring continued patient access to critical therapies.
What is the WISeR Model?
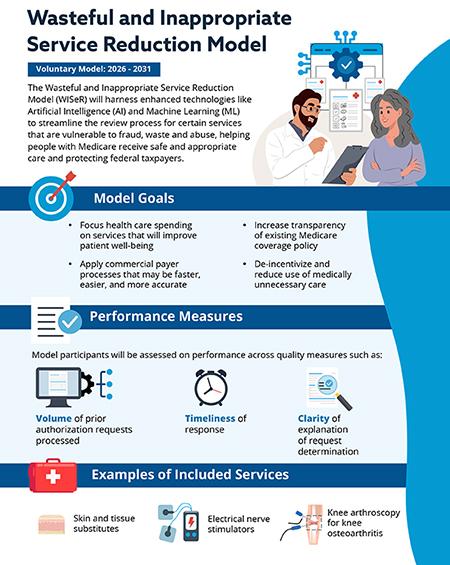
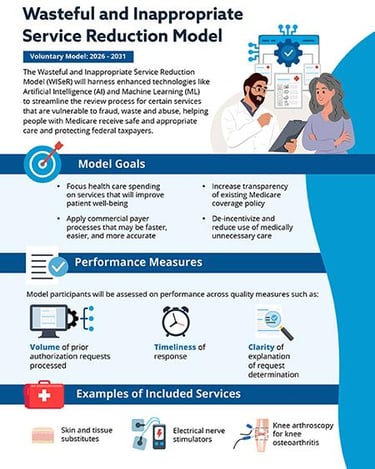
The WISeR Model is a mandatory, randomized clinical trial (RCT) conducted by the CMS Innovation Center (CMMI). Its primary goal is to test whether prior authorization for specific services, coupled with an innovative artificial intelligence (AI) and machine learning (ML) driven review process, can effectively reduce wasteful and inappropriate spending in Medicare Fee-for-Service (FFS) without negatively impacting beneficiary care [1, 2].
Key Components of the WISeR Model:
Targeted Services: WISeR focuses on a select list of Medicare Part B services that CMS has identified as being highly vulnerable to fraud, waste, and abuse. Crucially for the wound care community, skin and tissue substitutes (CTPs) are one of the primary targets [1, 3]. Other services include certain nerve blocks, specified minor surgical procedures, and some diagnostic imaging services.
Mandatory Prior Authorization: For providers in the selected intervention states, prior authorization will be required for the targeted services. This means that before rendering a service, providers must submit documentation to CMS or its contractors to demonstrate that the service meets Medicare's medical necessity criteria.
Artificial Intelligence (AI) and Machine Learning (ML): This is a distinguishing feature of WISeR. Instead of relying solely on human review, CMS plans to leverage advanced AI/ML algorithms to analyze prior authorization requests. These algorithms will learn from vast datasets of claims and medical records to identify patterns indicative of appropriate or inappropriate use. This is intended to streamline the review process for compliant requests while flagging those that require more intensive human review [1, 4].
Randomized Design: To rigorously test the model's effectiveness, the WISeR Model is structured as an RCT. Within each of the six intervention states, eligible providers will be randomly assigned to either an intervention arm (subject to prior authorization) or a control arm (continuing with existing claims processes) [1, 2].
Pilot States: The model will launch in six initial states: Arizona, New Jersey, Ohio, Oklahoma, Texas, and Washington. These states were chosen based on various factors, including high utilization rates of the targeted services [5].
Why Did the WISeR Model Come About?
The genesis of the WISeR Model lies in CMS's persistent efforts to combat escalating healthcare costs and ensure program integrity. Several factors converged to necessitate such an initiative:
Explosive Growth in Spending on Targeted Services: CMS has repeatedly highlighted the alarming increase in expenditures for certain services. For skin and tissue substitutes, spending escalated from approximately $256 million in 2019 to over $10 billion in 2024—a nearly 40-fold increase [6]. While some growth is expected with new technologies, CMS identified a significant portion as potentially wasteful or inappropriate.
Ineffectiveness of Post-Payment Review: Historically, CMS has relied heavily on "pay and chase" methods, reviewing claims for medical necessity after payment has been made. This approach is resource-intensive, often results in significant overpayments that are difficult to recoup, and creates administrative burden for providers facing audits and denials long after the service was rendered.
Precedent from Existing Prior Authorization Programs: CMS has seen success with prior authorization in other areas, such as power mobility devices and certain hospital outpatient services. These programs have demonstrated a reduction in improper payments and a shift toward more appropriate utilization [2]. The WISeR Model seeks to build upon these successes, applying lessons learned to new service areas.
Leveraging Advanced Technology: The advent of sophisticated AI and ML capabilities provides CMS with new tools to manage complex data. The agency aims to use these technologies to make prior authorization more efficient, less burdensome for providers who comply, and more effective at identifying problematic claims [4]. This is a strategic move to modernize program integrity efforts.
The Mandate of the Innovation Center: The CMMI is specifically tasked with testing innovative payment and service delivery models that can reduce expenditures while preserving or enhancing quality of care. WISeR is a direct fulfillment of this mandate [1, 2].
In essence, WISeR is CMS's proactive strike to "prevent and protect" rather than "pay and chase," leveraging technology to address services where waste and inappropriate use have been particularly rampant.
What to Expect from the WISeR Model?
For providers in the intervention states, the WISeR Model will introduce new operational complexities and demands for rigorous documentation.
1. Mandatory Prior Authorization for Targeted Services
For Skin Substitutes: If you are in Arizona, New Jersey, Ohio, Oklahoma, Texas, or Washington and your practice is randomly assigned to the intervention arm, you will need to obtain prior authorization for all applications of skin and tissue substitutes for Medicare Part B beneficiaries starting January 1, 2026.
Documentation Focus: Expect increased scrutiny on medical necessity. This means your clinical documentation must unequivocally support:
The chronicity of the wound (e.g., failure to heal after 30 days of standard care).
Adequate wound bed preparation (e.g., proper debridement).
Appropriate moisture balance and offloading measures.
The specific type of skin substitute chosen and its appropriateness for the wound type.
The rationale for repeated applications, if applicable.
Impact on Workflow: Prior authorization adds a step to the patient care pathway. Practices will need to dedicate staff and resources to submitting requests, tracking their status, and potentially appealing denials.
2. The Role of AI/ML
Faster Decisions for Compliant Requests: CMS hopes that AI/ML will allow for rapid approval of "green-light" requests that clearly meet medical necessity criteria, potentially reducing the turnaround time for straightforward cases.
Enhanced Scrutiny for "Red-Flag" Requests: Conversely, requests flagged by the AI/ML as potentially inappropriate or lacking sufficient documentation will be routed for more in-depth human review, which could lead to longer processing times or denials.
Learning and Adaptation: The AI/ML algorithms will continuously learn from submitted data. This means that over time, the system may become more sophisticated at identifying patterns of appropriate and inappropriate use.
3. Potential for Initial Disruptions
Learning Curve: Providers and their billing staff will face a learning curve in adapting to the new prior authorization requirements, submission portals, and documentation standards.
Impact on Scheduling: Delays in obtaining prior authorization could potentially impact patient scheduling and access to care, especially in the initial phases. CMS emphasizes that the model aims to prevent this, but it remains a potential challenge.
Denial Rates: Initial denial rates might be higher as providers adjust to the strict requirements, potentially leading to increased administrative burden for appeals.
4. Data Collection and Evaluation
Rigorous Monitoring: CMS will meticulously collect data on utilization, spending, quality of care, and beneficiary experience in both the intervention and control arms.
Future Policy Implications: The findings of the WISeR Model will inform future CMS policies regarding prior authorization for other services and the broader application of AI/ML in program integrity. If successful, elements of WISeR could be expanded nationally or to other service categories.
5. Collaboration and Communication
CMS Resources: Expect CMS to provide extensive educational materials, webinars, and technical assistance to help providers understand and comply with the model's requirements.
Industry Advocacy: Wound care industry associations and manufacturers will likely be actively engaged in providing feedback to CMS and assisting providers in navigating the changes.
The Combined Impact: WISeR and the CY 2026 PFS Rule
It's critical to view the WISeR Model not in isolation, but in conjunction with the CY 2026 PFS Final Rule's fundamental shift in skin substitute payment.
PFS Rule: Changes how much Medicare pays for a skin substitute application (by bundling the product into the procedure payment and effectively dismantling the high-margin "buy and bill" model).
WISeR Model: Changes how you get approval to even perform the service and receive that bundled payment (by requiring prior authorization and leveraging AI/ML).
Together, these two initiatives represent a dual crackdown on skin substitute spending. Providers are not only facing a significantly lower reimbursement margin but also a more stringent gatekeeping process to access that reimbursement.
This dual pressure necessitates a proactive and comprehensive strategy for wound management clinics and podiatry practices in the pilot states. A renewed focus on medical necessity, appropriate utilization, and robust documentation is paramount for successful navigation of this new regulatory landscape, ensuring continued access to limb salvage therapies for patients who truly need them.
See also
CMS Finalizes Historic Payment Overhaul for Skin Substitutes: What You Need to Know for 2026
The $10 Billion Question: How CMS's New Skin Substitute Crackdown Will Reshape the Wound Care Industry
When to Use Skin Substitutes or Grafts for Non-Healing Wounds
Stem Cells, Exosomes, and Biologics: Do They Work in Wound Care?
How to Tell If a Wound Is Healing: Signs of Proper Wound Care Progress
More Information
For more information on the latest effective wound care, contact us to set up a time for a call.
Sources
Medicare and Medicaid Programs; CY 2026 Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for 2026, Federal Register, November 5, 2025.
CMS Innovation Center – Wasteful and Inappropriate Service Reduction (WISeR) Model
Calendar Year (CY) 2026 Medicare Physician Fee Schedule Final Rule (CMS-1832-F), CMS Newsroom Fact Sheet, October 31, 2025.
CMS Proposes New AI Model to Reduce Wasteful Spending in Medicare, CMS Press Release, July 13, 2025.
CMS Modernizes Payment Accuracy and Significantly Cuts Spending Waste, CMS Newsroom Press Release, October 31, 2025.
Fact Sheet: Medicare Physician Fee Schedule
* This blog is for informational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment.
